Abstract
The relative clinical efficacy was compared between a chlorine dioxide
paste-rinse combination (test) regimen and a phenolic based rinse regimen (control) in a
blind, crossover study. Twenty Class II or III periodontal patients were ranomly assigned
as test or control subjects. Parameter measurements of plaque score, gingival bleeding,
gingival inflamation and pocket depth were recorded from the subjects at 0, 30 and 60 days
following prophylaxis. The test group demonstrated significant improvements with all
measured parameters except pocket depth when compared with controls. The mean bleeding
scores in the test and control group at were 0.727 and 0.750 respectively. At 60 days the
mean bleeding score was reduced to .427 in the control group and was 0.105 in the test
group. The mean gingival index scores were reduced from 1.105 and 0.911 at baseline in the
test and the control group to 0.561 and 0.733 respectively at 60 days. The mean plaque
scores were reduced from 1.344 to .766 in the test group as compared to 1.327 to 1.055 in
the control group. Probing depths were only slightly affected with means of 3.369 and
3.411 in the test and control groups which decreased to 2.950 and 3.144 respectively. The
reults suggest that the chlorine dioxide paste-rinse combination may have a greater
efficacy than the phenol related rinse regimen in improving three of four periodontal
parameters measured over 60 days.
Key Words: Halitosis, periodontal disease, gingival inflamation, volatile sulfur
compounds.
Introduction Periodontal researchers for years have sought for an
ideal plaque control agent. Ideally, the agent should be efficacious in reducing disease
activity and have breath freshening properties which enhance useage compliance. In the
last 15 years, only two compounds, chlorhexidine and triclosan, have been approved by The
United States Food and Drug Administration for plaque and gingivitis. Both compounds have
been studied extensively and have been shown to be efficacious for reducing plaque and
gingivitis. (1,2,3,4,5,6) Two chlorhexidine preparations have been accepted by The
American Dental Association for plaque and gingivitis. A phenol related rinse formulation
has also received acceptance from The American Dental Association for plaque and
gingivits, is widely commercially available and does not require a prescription.
(7,8,9,10,11,12) These compounds have also been used for the control of halitosis. (13)
Volatile sulphur compounds released by gram negative anaerobic bacteria from within the
periodontal pocket (14,15,16,17) and the dorsum of the tongue (13) have been isolated as
principle odorants. Tonzetich (18), using gas chromatography, isolated hydrogen sulfide,
methyl mercaptan and dimethyl sulfide in multiple mouth air samples from halitosis
patients. Yaegaki, et al., (19) reported that volatile sulphur compounds were
significantly elevated in periodontitis patients.
Although widely used for haltosis, the efficacy of stabilized chlorine dioxide has not
been extensively reported in controlled studies. Numerous anecdotal reports using
halimeters have shown reductions in measurable volatile sulphur compounds. Chemically,
chlorine dioxide oxidizes both hydrogen sulfide and methyl mercaptan via an
oxidation-reduction reaction. Therefore, in theory, because these compounds have been
chemically reduced, halitosis improved by a quantitative reduction of organoleptic agents.
There have been isolated reports in the literature of improved periodontal parameters
in gingivitis and periodontitis patients using stabilized chlorine dioxide for the control
of halitosis. Chapek, et al., (20) reported that 67.42 % of pockets in periodontal
maintenance patients measuring 4 mm or more had a measurable depth reduction at the next
recare appointment. The same group reported approximately a 72 % reduction of bleeding
upon probing in 239 refractory, bleeding pockets from one recare appointment to the
next.(21) Goultschin (22) showed a 34.50 % reduction in plaque scores when using a
stabilized form of chlorine dioxide as compared with controls when all other forms of oral
hygiene were suspended.
The purpose of this study was to investigate the relative efficacy on plaque level,
gingival index, bleeding upon probing and pocket depth of a stabilized chlorine dioxide
toothpaste and rinse regimen (a) as compared to a widely commercially available, phenol
related rinse regimen (b).
*Private Practice, Limited to Periodontics, Metairie, Louisiana.
**SGS Statistical Consulting Services, Tucson, Arizona
a. Oxyfresh Tooth Paste and Rinse, Oxyfresh Worldwide, Spokane, WA.
b. Listerine and Listerine Cool Mint Tooth Paste, Warner-Lambert Co., Morris Plains, N.J.
Materials and Methods: 20 patients were randomly assigned
into two groups as "test" , using the stabilized chlorine dioxide regimen, or
"control" using the phenol related regimen. The patients were all Class II or
III periodontal patients with 4 to 6 mm sites in a maintenance program. All patients had
been previously treated for periodontitis.
Exclusion criteria for participation in the study included the following:
1. significant cardiac histories, especially at risk for endocarditis 2. joint prosthesis
requiring antibiotic premedication 3. active cancer therapy 4. compromised renal function
5. infectious diseases (ie, HIV, tuberculosis, URTI) 6. systemic diseases- diabetes,
hepatic, lupus, etc 7. oral candidiasis 8. oral autoimmune/mucocutaneous diseases 9.
history of dilantin, cyclosporin, calcium channel blockers in past 12 months 10. systemic
antibiotics within last 3 months 11 NSAIDS within last 3 months 12 Chlorhexidine or
sanguinaria based products in last 3 months
Measurements- Site selection- All parameter sampling was done using the
"Ramjford" 6 teeth, which included the maxillary right first molar, the
maxillary right central incisor, the maxillary left first bicuspid, the mandibular left
first molar, the mandibular left central incisor and the mandibular right first bicuspid.
When a particular tooth was unavailable, an adjacent toooth was selected. 6 sites per
tooth were measured. All measurements were taken by a blind periodontist.
Baseline (day 0)- The parameter measurements taken were the Plaque Index (PLK
0-3), the Gingival Index (GI 0-3), Bleeding upon probing (BOP 0-3) and pocket depth (PD).
All patients received standard supportive periodontal maintenance procedures which
included suprgingival scaling and prophylaxis. All were instructed how to use the products
and were given written intructions with the products. These instructions were the same for
both groups except for the duration of the rinsing in the test group. Since stabilized
chlorine dioxide is activated by exposure to acids (H+) in the oral environment, the
manufacturer recommends rinsing for 90 seconds. The controls were advised to rinse for at
least 30 sec. with the phenolic related rinse. The subjects were instructed to use the
respective products exclusively for the duration of the study, to do so twice each day and
to record their compliance on a reporting form. The same measurments were repeated at 30
Days and at 60 days. All patients were re-examined by the same periodontist who was
blinded as to which products the patient was using. All four parameters were re-examined.
Patients were re-screened for exclusion criteria.
Data Analysis- The data were grouped by measuments type and subjected to
analysis of variance (ANOVA) for diferences between the groups using a commercially
available statisical computer package (c).
c. SAS, SAS Institute, Cary, NC.
Results- All patients completed the duration of the study.
Compliance with the prescribed oral hygiene regimen was reported by each participant and
measured as a percent. The compliance of the test group was 99.2% and was 99.1% in the
control group.
The results for each parameter are shown in table 1.
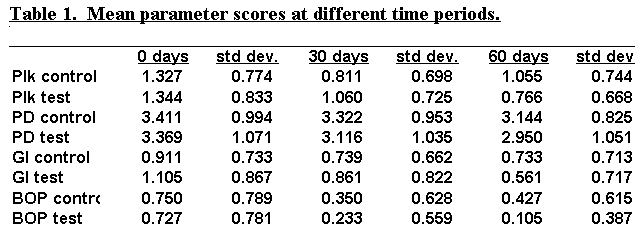
The mean bleeding scores were similar in the test and control group at
baseline and measured to be 0.727 and 0.750 respectively. At 60 days the mean bleeding
score was reduced to .427 in the control group and was nearly eliminated as reflected by
the score of 0.105 in the test group. These findings were significant as P<.001. See figures 1 and 2.
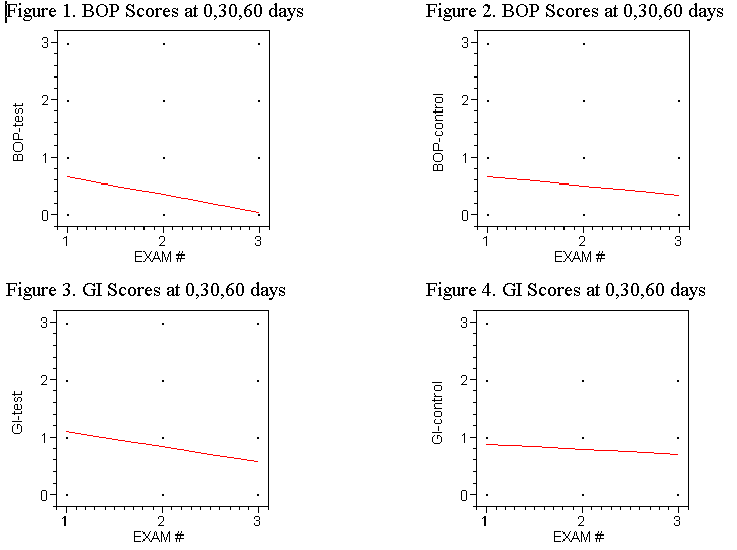
Similarly, the
mean gingival index scores were reduced from 1.105 and 0.911 at baseline in the test and
the control group to 0.561 and 0.733 respectively at 60 days. These findings were
significant as P<.001. See figures 3 and 4.
Plaque Scores were reduced from 1.344 to.766 in the test group as
compared to 1.327 to 1.055 in the control group as shown in Figures 5 and 6. These
findings were significant as P<.001.
Finally, probing depths were only slightly affected with
means of 3.369 and 3.411 in the test and control groups which decreased to 2.950 and 3.144
respectively. These findings were significant as P<.001. See figures 7 and 8.
<.001
Discussion The results of this study clearly demonstrate the
efficacy of both paste- rinse protocols. The use of both products resulted in significant
reductions in the plaque score, bleeding upon probing and the gingival index.
Very limited changes were noted with respect to probing depths. The lack of
significance was expected as clinicians rarely note probing depth reductions due to the
use of topically applied antiplaque agents.
The results of this study compare favorably with the work of other reports,
particularly with respect to reports addressing the efficacy of phenolic based rinses.
Previous reports have demonstrated plaque reductions varying from 20 to 34% as compared
with 20.5% in this study. Gingivitis reductions have been reported in the same range as
compared to 19.5% in this study. (17,18,19)
The use of the phenolic regimen was included as a positive control in the crossover
study design. The phenolic control was used since it is available as both a toothpaste and
a rinse in The United States, unlike chlorhexidine. Since the test product performed
better than the positive control, a relative efficacy can be established using the dual
paste-rinse regimen. The efficacy of stabilized chlorine dioxide has not been previously
reported as compared to placebos or other positive controls.
The duration of exposure to the active ingredients significantly differed between the
test and control groups. In the test group, each subject was exposed to chlorine dioxide
for approximately 120 seconds during brushings and 90 seconds during rinsing two times per
day. In the control group, the subjects were only exposed to the phenol related essential
oils for 30 seconds twice each day. The duration of exposure to the active ingredients may
have a significant affect on the improvements seen, favoring the chlorine dioxide group.
This results of this study are based upon normal home use of the products according to
the manufacturers instructions and coupled with specific intructions on proper tooth
brushing, flossing and rinsing. Additional controlled studies should separate the paste
from the rinse to determine if the sole use of either can generate clinical improvements.
Also, controlled studies of stabilized chlorine dioxide alone, divorced from the benefits
of tooth brushing and flossing would provide additional useful information.
In conclusion, both the stabilized chlorine dioxide tooth paste and rinse regimen and
the phenol related rinse regimen resulted in measurable clinical improvements with respect
to gingival bleeding, gingival inflamation and plaque scores during the 60 day obervation
period. These findings suggest that there may be significant periodontal benefits in using
a stabilized chlorine dioxide tooth paste and rinse regimen.
References