Periodontal Specialist Local
Drug Delivery for the treatment of periodontitis is again being revisited and this time it
is by Chlorhexidine. No other product has been approved for this use since Actisite. In
1998, The FDA granted approval for The PerioChip which is marketed by Astra, Inc..
This product is generating a lot of interest. New innovations
are always exciting, especially if they perform in a manner consistent with all the
hype. Unfortunately, many do not. I have received so many calls from dental
colleagues asking what I thought of the PerioChip. . The answer is ......
Each chip contains 2.5 mg of chlorhexidine in a biodegradable matrix of hydrolyzed
gelatin. The chip measures 4 x 5 x 1 mm. It is supposed to be placed
into a periodontal pocket following root planing. The chip slowly dissolves by a
hydrolysis reaction and should be completely gone in 10 days. The bulk of the
chlorhexidine 40 % is released within 24 hours and the balance over the remaining life of
the chip. Therefore, you do not have to bring the patient back for follow-up
(however, follow-up is usually beneficial in patients with periodontitis so that proper
oral hygiene can be reinforced).
The chips are sold in boxes of ten for $160.00. If you
buy in quantity, the unit cost comes down. One chip is good for one pocket. If
you have a tooth with pocketing on three surfaces, you will need three chips. The
manufacturer does not advise trying to cut the chip because you will reduce the total dose
of chlorhexidine released into the pocket. The improvements seen are dose dependent.
Let's take a look at this product and evaluate it's clinical
performance compared to Actisite when placed into a periodontal pocket combined with
scaling and root planing as compared to scaling and root planing alone. To do this,
we have to examine the literature behind each treatment mode.
I have prepared the following three tables from the results shown in
pertinent studies with respect to reduction in pocket depth and bleeding upon probing and
the gain in clinical attachment levels.
Three landmark studies investigating local drug
delivery vs. root planing were done by Newman, et al, J. Perio 65:685, 1994 and Drisco, et
al, J Perio, 1995 for tetracycline fibers. Solskone, et al, J Perio, 68:32, 1997
reported on chlorhexidine chips.
Legend: All measurements are reported in millimeters.
RP=root planing alone, RP/LD=root planing with local drug delivery, Diff=the
difference between the two treatment groups, @= approximate measurement taken from data
plots in the publication (the actual numeric data was not specified).
Pocket Depths
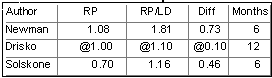
Bleeding Upon Probing

Gain in Clinical Attachment Level
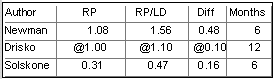
As you can see the reported numbers findings for the three treatment
modalities are very similar. In the Newman study and also in the Solskone study,
local drug delivery generated improvements for the three parameters which were claimed to
be statistically significant. The findings in the Drisco study were that fibers with root
planing were not statistically better than root planing alone.
My interpretation of these results is as follows:
I have to question how significant is a statistically significant improvement in
clinical attachment level of 0.48 mm in the Newman study and 0.16 mm in the Solskone
study? Are the findings significant in a clinical sense? The American Academy
of Periodontology wrote in a statement to all AAP members, "The magnitude of these
changes are very small. Furthermore, it should be noted that these are limited
alterations in patients with advanced periodontitis." In my opinion, local drug
delivery has a long way to go to generate results that are clinically significant.
Do the results hold up over time? Longer
term studies of the chip need to be done to answer these questions. If you
extrapolate the results from longer term Actisite studies, assuming that chip treated
sites will respond like the Actisite sites, then we can expect a slow reopening of the
pocket. The reason for this trend has nothing to do with the local drug delivery
system. It is very simply due to our clinical limitations in closed root
debridement.
Numerous studies have shown that as the pocket depth increases, our
ability to thoroughly debride the root decreases. Most clinicians would be surprised
to find that most pockets of 5 mm or more have residual calculus following root
planing. Additionally, root anomalies, such as root flutes, developmental grooves,
furcation invasions and enamel projections further diminish our debridement
efficacy. These nidus' of subgingival plaque gradually repopulate the immediate area
of the sulcus and cause the pocket to reform.
No studies have been published to date which
investigate the efficacy of the chip in the following circumstances: localized juvenile
periodontitis, rapidly progressive periodontitis, furcation lesions and intrabony defects.
In summary, I would propose the
following guidelines:
1. you should expect results only comparable to
Actisite.
2. You should use the chip in refractory sites (i.e., those which have not
responded to root planing alone after you have allowed a sufficient time period to
reevaluate the site).
3. You should avoid trying them in pockets greater than 7 mm or sites with root
anomalies and furcation lesions. Using them in areas of simple root morphology will
result in improved results.